Difference Between Simple And Fractional Distillation & Diagram
Simple distillation or simple distillation is a type of distillation where the vapors produced are channeled to a condenser, this results in a poorly pure distillate.
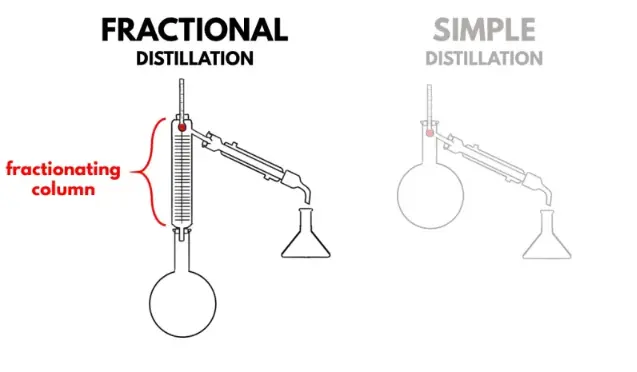
Fractional distillation is a process in which a fractionation column is inserted between the flask and the distillation head that allows greater contact between the vapors that ascend with the condensed liquid that descends, achieving a much purer distillation.
Simple And Fractional Distillation Difference
Simple distillation separates components with significantly different boiling points, while fractional distillation separates components with closer boiling points by utilizing a fractionating column to enhance separation efficiency. Fractional distillation is more effective for refining complex mixtures like petroleum, while simple distillation is suitable for purifying liquids with distinct boiling points.
Simple Distillation Method:
During this process, the vapor produced is channeled to a condenser, which cools it and brings it to a liquid state, resulting in a non-pure distillate. It is used to separate mixtures of liquids whose boiling points differ by up to 80ºC or to separate liquids from non-volatile solids.
Equipment known as an alembic is used ; it consists of a container in which the mixture is heated, a condenser that cools the vapors, and a container that stores the distillate.
In chemistry it is used to separate simple or complex mixtures.
It consists of two stages:
- The mixture or solution is brought to a boil to vaporize the most volatile component of the mixture, that is, the one with the lowest boiling point.
- The vapor passes from the flask to the condenser, where it is cooled by cold water and changes from a gaseous state to a liquid state; This is collected in the collecting flask.
Fractional Distillation Method:
This technique allows a series of simple distillations to be carried out in a single operation. It is used to separate liquid compounds that differ by less than 25ºC in their boiling point.
It is a setup similar to that of simple distillation, but a fractionation column containing rings, helices or plates is inserted between the flask and the distillation head. This process is widely used in refineries to obtain petroleum derivatives.
The fractionation column allows greater contact between the vapors that ascend, with the condensed liquid that descends through the use of different plates or plates.
This facilitates heat exchange between vapors and liquids; This process facilitates a mass exchange, where liquids that have a lower boiling point are converted to vapor and vapors of substances with a higher boiling point become a liquid state.
Summary & Key Points About Simple And Fractional Distillation:
- In simple distillation, a liquid is boiled and the vapors produced are directed to a condenser where it will pass into the liquid state.
- In fractional distillation, a fractionation column is used that forces the vapor to cool and return to the container, causing contact with the liquid to boil again. The gas then travels and is retained in the different plates of the fractionation column.
- In simple distillation, the liquid boils at a higher temperature than that required for fractional distillation.
- Simple distillation can be applied in mixtures with less than 10% impurities.
- Mixtures with more than 10% impurities require fractional distillation.




