Thermal Equilibrium: Definition And Examples
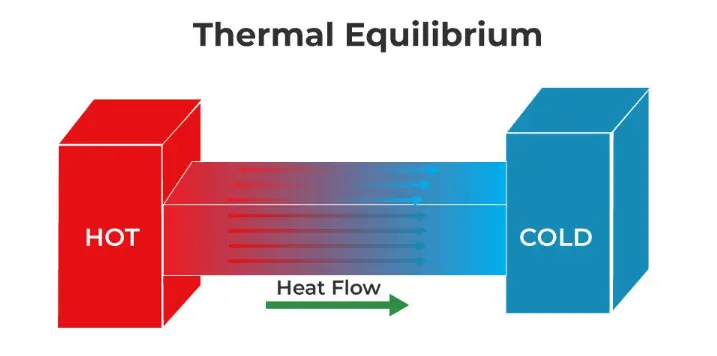
When two bodies that are at different temperatures are brought into contact, the hotter one gives up part of its energy to the one with a lower temperature, up to the point at which both temperatures are equal.
This situation is known as thermal equilibrium, and it is precisely the state in which the temperatures of two bodies that initially had different temperatures are equal. It happens that when the temperatures equalize, the heat flow is suspended, and then the equilibrium situation is reached.
Theoretically, thermal equilibrium is fundamental in what is known as the Zeroth Law or the Zeroth Principle of Thermodynamics, which explains that if two separate systems are at the same moment in thermal equilibrium with a third system, those are in thermal equilibrium one with another. This Law is fundamental for the entire discipline of thermodynamics, which is the branch of physics that deals with describing equilibrium states at a macroscopic level.
Equilibrium heat quantity
The equation that gives rise to the quantification of the amount of heat exchanged in transfers between bodies has the form:
Q = M * C * ΔT
Where Q is the amount of heat expressed in calories , M is the mass of the body under study, C is the specific heat of the body, and ΔT is the temperature difference.
In an equilibrium situation, the mass and specific heat retain their original value, but the temperature difference becomes 0 because it was precisely defined to be the equilibrium situation where there are no temperature changes.
Equilibrium temperature
Another important equation for the idea of thermal equilibrium is the one that seeks to express the temperature that the unified system will have . It is accepted that when a system of N1 particles, which is at temperature T1, is brought into contact with another system of N2 particles that is at temperature T2, the equilibrium temperature is obtained by the formula:
(N1*T1 + N2*T2)/(N1+N2)
In this way, it can be seen that when both subsystems have the same number of particles, the equilibrium temperature is reduced to an average between the two initial temperatures. This can be generalized to relationships between more than two subsystems.
Examples of thermal equilibrium
Below are some examples of situations where thermal equilibrium occurs:
- Measuring body temperature through a thermometer works that way. The prolonged duration that the thermometer must have in contact with the body to be able to truly quantify the degrees of temperature is due precisely to the time it takes to reach thermal equilibrium.
- The products that are sold ‘naturally’ could have passed through a refrigerator. However, after a certain amount of time outside the refrigerator, in contact with the natural environment, they reached thermal equilibrium with it.
- The permanence of glaciers in the seas and at the poles is a particular case of thermal equilibrium. Precisely, the warnings regarding global warming have a lot to do with an increase in sea temperature, and then a thermal balance where much of that ice melts.
- When a person comes out of bathing, he or she is relatively cold because the body had come into balance with the hot water, and now must come into balance with the environment.
- When you want to cool a cup of coffee, adding cold milk.
- Substances such as butter are very sensitive to changes in temperature, and with very little time in contact with the environment at natural temperature they come into equilibrium and melt.
- When you put your hand on a cold handrail, for a time, the hand becomes colder in temperature.
- A jar with a kilo of ice cream will melt slower than another with a quarter kilo of the same ice cream. This is produced by the equation in which mass determines the characteristics of thermal balance.
- When an ice cube is placed in a glass of water, thermal equilibrium also occurs. The only difference is that equilibrium implies a change of state, because it crosses 100°C where water goes from solid to liquid.
- Adding cold water to a rate of hot water, where equilibrium is very quickly reached at a temperature colder than the original.