Difference Between Alloy AND Composite
In this article, you will learn what is the basic difference between alloy and composite. How they are formed, what they have different properties, and much more.
The basic difference between alloy and composite is that an alloy is a homogeneous mixture of two or more metals, while a compound is a chemical combination of two or more elements in defined proportions.
Alloys are formed through physical processes, such as melting and solidification, while compounds are formed through chemical bonds.
Alloys have different properties than pure metals, while compounds exhibit unique distinctive properties.
Difference Between Alloy and Composite:
What is an Alloy..?
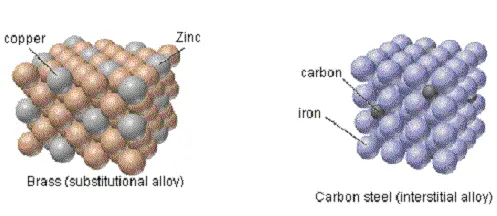
An alloy is a homogeneous mixture of two or more metals, or one metal with another element, created by melting or solidifying the components.
In an alloy, the atoms of different metals are dispersed within the crystal structure, resulting in a material with different properties than pure metals.
These properties may include increased mechanical strength, malleability, and corrosion resistance.
Alloys have been used for centuries for their ability to improve the properties of metals and adapt to various industrial applications.
Give the 5 Examples of Alloy:
Alloys are mixtures of two or more elements, where at least one of the components is a metal:
- Bronze
- Steel
- Brass
- Stainless Steel
- Alnico
What is A Compound..?
A compound is a chemical combination of two or more different elements in defined proportions.
Unlike alloys, compounds are formed by chemical bonds between the atoms of the constituent elements.
These bonds can be covalent or ionic, resulting in a unique molecular structure. Compounds exhibit distinctive properties that are different from those of the elements that compose them.
For example, water (H2O) is a compound formed by two hydrogen atoms and one oxygen atom, and has properties such as the ability to dissolve many substances and a high surface tension.
Give the 5 Examples of Compound:
Compounds are substances formed when two or more elements chemically combine in fixed proportions:
- Water (H2O)
- Table Salt (Sodium Chloride, NaCl)
- Carbon Dioxide (CO2)
- Methane (CH4)
- Calcium Carbonate (CaCO3)
Comparative Table
Alloy Vs Compound:
| Characteristic | Alloy | Compound |
|---|---|---|
| Composition | Mixture of two or more metals | Combination of elements |
| Union of components | Fusion or solidification | Chemical bond |
| Properties | Stronger and more malleable | Distinctive properties |
| Common examples | Steel, bronze, brass | Water, table salt |
Conclusion
Alloys and compounds are fundamental terms in the study of materials. Alloys are mixtures of metals or metals with other elements, while compounds are chemical combinations of elements in defined proportions.
Alloys are formed through physical processes, such as melting and solidification, while composites are formed through chemical bonds.
The properties and characteristics of alloys differ from those of pure metals, providing greater strength and malleability.
Compounds, on the other hand, exhibit distinctive properties that are different from those of the constituent elements.
By understanding these differences, we can better appreciate the complexity and diversity of the materials around us and harness their potential in various technological and industrial applications.
You May Also Like:
- Difference Between Astronomy And Astrology
- Difference Between Anatomy And Physiology
- Difference Between Axiology And Ethics
- Difference Between Aurora Borealis And Australis
- Difference Between Atheists and Agnostics

